Ph Value Calculation
HCO3 calculation is also provided some blood gaz machines do not provide this result. Here we are going to calculate pH of 01 mol dm-3 aqueous ammonia solution.

How To Calculate The Ph Of A Solution Youtube
However it is only an approximation and should not be used for concentrated solutions or for.

. Here we will study the pH value formula and how pH value is calculated in detail. In other words pH is the negative log of the molar hydrogen ion concentration or the molar hydrogen ion concentration equals 10 to the power of the negative pH value. Its easy to do this calculation on any scientific calculator because more often than not these have a log button.
PH pH at 55ºC of the sample solution obtained in Identification 1 is 38 - 76. The quantitative behavior of acids and bases in solution can be understood only if their pKa values are known. Current directory otherwise.
1 To recover part of the phx calculation even if the recover file or files are corrupted. Ie Efficiency of the pump is the ratio water horse power to break horse power. 2 Iron - To 500 g of Gelatin in a glass-stoppered.
The Henderson-Hasselbalch equation relates pKa and pH. The expected pH is an estimation of the pH that would be observed if pCO2 were the only abnormal value. One of the outstanding mysteries surrounding the rich diversity found in supernova remnants SNRs is the recent discovery of over-ionized or recombining plasma from a number of dynamically evolved objects.
Store Sautéed Cabbage in an airtight container and place in the refrigerator for up to one week. Pump input power calculation formula or pump shaft power calculation formula. Cost of capital is the required return necessary to make a capital budgeting project such as building a new factory worthwhile.
Purity 1 Heavy metals - Proceed with 05g of Gelatin according to Method 2 and perform the test. If the molarity of an aqueous solution is 63 10-5 M what is the pH. Calculate pH of ammonia by using dissociation constant K b value of ammonia.
To help decipher its formation mechanism we have developed a new simulation framework capable of modeling the time evolution of the ionization state of the. The lower the pKa the stronger the acid and the greater the ability to donate a proton in aqueous solution. The pH scale which normally spans from 0 to 14 in water is used to determine the pH of an aqueous solution.
This is not the same as the ln button which refers to the. The pH scale ranges from 0 to 14 under usual conditions and measures the acidity of an aqueous solution. To be used for the evaluation of the respiratory component of an acidosis alkalosis.
Place Sautéed Cabbage in a microwave-safe bowl or on a plate and reheat gently until warmYou can perk your leftovers up by adding another sprinkle of salt and a splash of apple cider vinegar after reheating. Consider use of incremental FiO 2PEEP combinations such as shown below not required to achieve goal. The calculation becomes much more complicated and you will need to solve.
Dissociation constant K b of ammonia is 18 10-5 mol dm-3Following steps are important in. Of the molarity reverse the sign to get a positive value and youre done. An activity coefficient is a factor used in thermodynamics to account for deviations from ideal behaviour in a mixture of chemical substances.
PKa - The pKa value is the negative base -10 logarithm of the acid dissociation constant Ka of a solution. You just remove the _ph0prefixrecover files from the tmp_dir directory. The pH value can be less than 0 for very concentrated strong acids or greater than 14 for very concentrated strong bases.
PaO 2 55-80 mmHg or SpO 2 88-95 Use a minimum PEEP of 5 cm H 2O. Heat energy also called thermal energy or just heat is transferred from one location to another and temperature is a measurement of that energy. To learn more about Calculation of pka List of pKa values Relationship between pKa and pH and FAQs of pKa Visit BYJUS.
You can also remove all the _ph0 files and keep only the _ph0prefixphsave. The pKa is the pH value at which a chemical species will accept or donate a proton. A pH of more than 7 is classified as basic.
Pump Input Power P. Cost of capital includes the cost of debt and the cost of equity. T and RR to achieve pH and plateau pressure goals below.
Hydraulic power Ph Flow rate X Total developed head X Density X Gravitational constant. Acidity is defined as a pH of less than 7. In an ideal mixture the microscopic interactions between each pair of chemical species are the same or macroscopically equivalent the enthalpy change of solution and volume variation in mixing is zero and as a result properties of the.
A pH of 7 is regarded as neutral. Pump Efficiency is the ratio of pump input and output power. Value of the ESPRESSO_TMPDIR environment variable if set.
The calculation of the pH of a solution containing acids andor bases is an example of a chemical speciation calculation that is a mathematical procedure for calculating the concentrations of all chemical species that. Prepare the control solution with 25 mL of Standard Lead Solution not m50.

Ph Poh H3o Oh Kw Ka Kb Pka And Pkb Basic Calculations Acids And Bases Chemistry Problems Youtube

How To Calculate The Ph Of A Strong Base Solution Chemistry Study Com

How To Calculate Ph From The Hydrogen Ion Concentration Chemistry Study Com
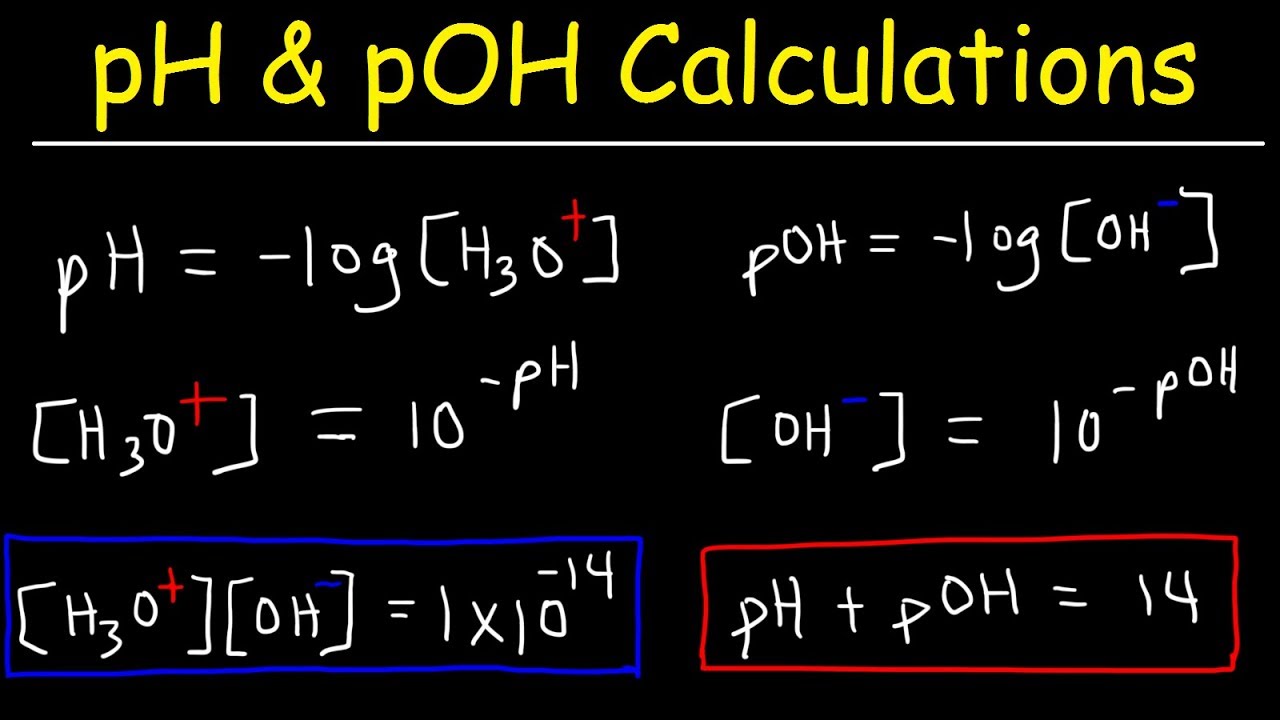
Ph Poh H3o Oh Kw Ka Kb Pka And Pkb Basic Calculations Acids And Bases Chemistry Problems Youtube
No comments for "Ph Value Calculation"
Post a Comment